
Copyright © 2005 the Brewery History Society
|
Journal Home > Archive > Issue Contents > Brew. Hist., 121, pp. 40-46 |
A Chapter in the History of Transfer of Information on Attenuation |
by Mikuláš Teich |
Due to formation of alcohol during fermentation the specific gravity of wort falls. This phenomenon is known as ‘attenuation’ and its importance for the control of the course of fermentation was certainly appreciated in Britain by John Richardson by 1784. In his pathbreaking Statical Estimates of the Materials of Brewing he writes:
The attenuation of a given weight of fermentable matter, in any fluid, will produce a certain quantity of spirit (alcohol-M.T.), and that equal quantities of attenuated matter, in all fluids , whether of equal or different densities, will produce equal quantities of spirit, without any regard to the proportion which such attenuation may bear to the density of either.
To emphasize the point, Richardson had this passage printed in italic type.1
The history of measurement of specific gravity goes back to Archimedes who initiated the study of fluids at rest. The ‘hydrostatic instrument’ by means of which the specific gravity of a liquid was determined became known as ‘hydrometer’. It was Richardson's historical merit that he realized the potential of hydrometric measurements for producing uniform beers by registering changes in the specific gravity of wort during fermentation. Accordingly, he states:
If the specific gravity of the fluid be noted immediately before fermentation, and again at any time after, when the operation has entirely ceased, the difference between the former and the latter will indicate the weight of fermentable matter attenuated, and, of course, the quantity of spirit produced.2
The fermentable matter in wort, the sweet liquid drawn from the mash tun, was known to be ‘saccharine’ or sugary. Whence Richardson called the hydrometer adapted to measure its specific gravity ‘saccharometer’. The term as such appears in the fuller version of the title of the book as An instrument constructed for the purposes of regulating to advantage the oeconomy of the brewhouse; and of establishing the means of producing uniform strength in maltliquors.
Richardson pointed out that while there was apparent correlation between the amount of alcohol in beer (‘strength’) and attenuation, it was not precise.3 A deeper understanding of attenuation resulted from the work of two Edinburgh university professors T. Ch. Hope (Chemistry) and A. Coventry (Agriculture) and T. Thomson who held a Lectureship at the University. They were asked by the Board of Excise for Scotland to ascertain the relative qualities of malt from barley and Scotch bigg. The latter was regarded as an inferior grain and therefore, it was suggested, the duty on malt from Scotch bigg should be lowered. The findings by the Edinburgh investigators were published as Parliamentary Papers in 1806.4
What interests us here is that they were critical of the view held by distillers that the common saccharometers indicated the quantity of extract that disappeared:
because all of them are constructed upon the principle, that the degrees of their scale should correspond with, and indicate a certain quantity of extract contained in a given bulk of wort, while none of them exhibit the real specific gravity of the fluid; and in no case has the relation between their degrees, and the real specific gravity, been determined. Hence, these instruments, though they manifest a progressive change in the density of worts, and in so far are very valuable, do not indicate the true specific gravity, nor the change of it. To remedy their imperfections, one of our number, Dr. Thomson, has constructed a saccharometer which exhibits the real specific gravity, as hydrometers do. In it the zero, or beginning of the scale, represents the specific gravity of distilled water, which is called one thousand, and each degree is equivalent to 1/1000th part. By the assistance of a sliding rule, the real specific gravity may at once be translated into the language of the Distillery, and it then denotes the quantity of extract per barrel, and also the proportion of extract per cent. in any wort. This instrument, therefore, indicates the real specific gravity and truly shews the attenuations ...5
With the aid of this saccharometer, the three reporters spent much effort upon comprehending the course of fermentation whereupon they concluded that is was determined by two opposite processes:
The change of specific gravity proceeds from two causes of a very contrary nature, which conspire, however, to produce the same effect. The one is the actual decomposition, and consequent decrease of the solid matter of the extract, and the other the substitution of the very light substance of alcohol. These always keep pace with each other, and by their joint effect increase the ultimate attenuation. Whenever any portion of spirit is generated, in consequence of its levity, it counteracts the gravity of the remaining unchanged extract, and causes the fermenting wort, or wash, to appear less dense than from the quantity of extract it otherwise would do. This circumstance is the source of the mistake above-mentioned, into which Distillers often fall, and renders the indication of the saccharometers, respecting the quantity of extract remaining in wash, and the conclusions of Distillers concerning the quantity of it consumed, altogether erroneous.6
Important as these findings were they were not immediately assimilated into brewing. It took 37 years before Carl Joseph Napoleon Balling, who taught at the Polytechnical Institute in Prague, perceived their significance. Before we come to this, it may be worth to recall Bohemia's and Moravia's (Czech Lands) marked place in the development of Continental brewing. Not merely because Moravian barley and Bohemian hops as raw materials have long enjoyed international reputation.
It has been largely forgotten that a sixteenth-century Czech polymath published in 1585 in Frankfort/Main a small book on brewing: De cerevisia eiusque conficiendi ratione natura, viribus et facultatibus opusculum.7 It was authored by Tadeáš Hájek z Hájku, known as Hagecius, or Nemicus. He eventually became a personal physician of Emperor Rudolph II and Chief Medical Officer of Bohemia.8 It appears that the suggestion to Hagecius to write the booklet came from another personal physician of Rudolph II. Whereas its medical and pharmaceutical dimension does not concern us,9 Hagecius' steps to get acquainted with brewing practice do. Ignorant as he was of it, he consulted with humble brewers who provided him - he acknowledges - with full information even though it was simple and unsystematic. Poignantly, he regarded production of beer as a legitimate field for scientific inquiry and rejected the notion that it was an undignified scholarly pursuit. Clearly, Hagecius did not perceive the worlds of intellectual and manual labour to be separated by an impervious wall.
Hagecius died in 1600, 18 years before the defenestration of two highest state officials (and a clerk) from Prague Castle which famously marks the beginning of the ruinous Thirty Years War. It took about two postwar generations before brewing in Bohemia began to recover, the trend accelerating after 1788 due to considerable liberalisation of the trade. As it happened, it was in this year that a self-taught Czech brewer, František Ondrej Poupe (Franz Andreas Paupie) began to employ the thermometer which, even in England, was not standard practice at that time.10 He was first to bring not only the thermometer but also the hydrometer into systematic use in brewing in the Czech Lands. He gave an account of his views on brewing in three books.11 The reasons which led Poupe to introduce scientific elements of control into brewing are set forth in the 31st chapter of the second edition of Die Kunst des Bierbrauens. Already the heading of this chapter indicates that he gained his knowledge as a practising brewer.12
As in England, the use of thermometer and hydrometer was resisted by conservative brewers. Poupe compared the brewer who did not use these instruments to a seafarer who went to sea without a compass. Poupe used the thermometer during all stages of beer brewing. This was an important break with traditional procedure which estimated temperatures merely empirically. For the determination of specific gravity of liquids Poupe employed a special type of hydrometer or beer scale made of brass. From the values determined by it Poupe inferred the strength of beers. This is surprising because he was aware that this procedure was not justified.
In this connection he referred to Christian Polykarp Friedrich Erxleben who about the same time was also constructing a hydrometer for the use of brewing. Erxleben, a pharmacist and industrial entrepreneur of German origin - settled in Bohemia - undoubtedly surpassed Poupe as a theoretician. This can be confirmed by studying his publication on beer, in which he discussed the problem what constituted ‘goodness’ and ‘strength’ of beer.13
Erxleben believed that the strength of beer was determined by the amount of alcohol contained in it and he strove hard to demonstrate that the latter could be quantitatively determined only after separating it by distillation, and not by the hydrometer. This instrument, he thought, was to be employed to detect adulteration of beer to which water was added. Clearly, Erxleben was aware of attenuation. His efforts to come to terms with this phenomenon belong to the history of introducing science into brewing practice. It would seem that it was his detailed knowledge of brewing which enabled Erxleben to break new ground in the understanding of fermentation. That is, he explicitly suggested that fermentation has to be viewed as much in chemical as in biological terms.
How did Erxleben arrive at this novel notion of the nature of fermentation - not appreciated at that time? He started from the supposition that the preparation of malt was only partly under the control of man, with the result that the brewer had to make use of diverse malts. Erxleben traced the cause of this variability to plant growth: the germination of the barley used resulted in non-uniform brewers' malts. Accordingly, Erxleben maintained worts brewed from them would necessarily be of changeable composition so the specific gravity of the beer could not be expected to be uniform, even if the fermenting procedures used were always the same, which led him to state:
As a rule one can indeed state in advance with certainty the result of any once known chemical operation, but here an exception occurs. Because the fermentation, although until now always considered as such, appears in no way a mere chemical operation but much rather in part a process by which plants grow, and must be considered as the link in the great chain in nature which brings about union of the activities which we call chemical processes with those of plantlike growth.14
Erxleben's work influenced Balling who studied attenuation experimentally. How Balling got involved into studying the phenomenon of attenuation constitutes a notable exemplar of scientific-technical diffusion in the nineteenth century.
The Polytechnical Institute in Prague was established in 1806. Remarkably, by 1818 J.J. Steinmann, the Professor of Technical Chemistry, started to give lectures on brewing. Balling, who succeeded Steinmann in 1833, was asked repeatedly by Excise for Bohemia and Austria to give advice on beer and spirits taxation. Because he knew next to nothing about these matters, he began to fill the gap in his knowledge by reading intensively. It was in this way he came across 1833 the German version of the Hope-Coventry-Thomson Report, which appeared 1822 in the journal Der deutsche Gewerbsfreund with a footnote by its editor K.W.G. Kastner stressing its scientific value. While the Report found no echo in German scientific and brewing circles, Balling perceived immediately the significance of the information contained in it. As he wrote in his first important statement on the subject:
[The Report] is very instructive; here I learned about employment of the word attenuation for the lowering of specific gravity, which sugary fluids experience during alcoholic fermentation. And I also accepted this exceedingly good term and employed it continuously since then in my publications dealing with matters relating to fermentation chemistry.
But then he went on to say:
But neither the existing books on brewing nor that Report offered complete information which I needed and desired. And thus I was prompted to undertake experiments. Because they were interesting, I persevered and broadened their scope since that time and thus acquired a good deal of valuable and useful knowledge regarding fermentation chemistry as a whole.15
Ten years after Balling received the first impulse to study attenuation, he published the cited Die saccharometrische Bierprobe (1843). Although it contained only about 50 pages, it may be justifiably regarded as a milestone in the development of fermentation practice on a scientific basis.
It provided a guide to rational control of the fermentation process. Balling visited breweries and distilleries in order to get first-hand information about fermenting. He also conducted large-scale experiments of his own within the precinct of the Polytechnical Institute. Balling mentions 100 experiments in fermentation for making beer and spirits and about 1000 investigations of obtained worts. He invented a saccharometer which expressed the wort strength as percentage of sugar, assuming that the wort is substantially a solution of cane sugar (‘degree Balling’). By means of this device he measured the extract of the original wort (O), the apparent extract of the beer (Ea) and the real extract of the alcohol-free beer (Er). They served him to designate
These relations became the subject of Balling's experimental investigations which led him to compose a table of ‘alcoholic factors’ that enabled him to establish the alcoholic content by weight (A). Without going into detail the formula which came into use - following Balling - was
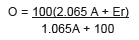
A product and a factor of transition to industrial brewing, Balling's ‘Attenuationslehre’ became an avenue by which science affected brewing practice seriously in Bohemia, Austria and Germany since about 1850. The article draws on historical studies in which I have been involved. See my book Bier, Wissenschaft und Wirtschaft in Deutschland 1800-1914 (Vienna, 2000).
|
1. |
Richardson, J. (1784) Statical Estimates of the Materials of Brewing; etc. (London), 27-8. |
|
2. |
Richardson, J. ibid. 28. |
|
3. |
For a welcome critical study of Richardson's contribution to eighteenth-century theory and practice of brewing in the English context, see Sumner, J. (2001) ‘John Richardson, saccharometry and the pounds-per-barrel extract: the construction of a quantity’, The British Journal for the History of Science, 34, 255-73. |
|
4. |
Papers Presented to the House of Commons Relating to Experiments … to ascertain the relative qualities of malt made from barley and Scotch bigg; etc. Ordered to be printed 6th June 1806. |
|
5. |
Ibid., 66. |
|
6. |
Ibid. |
|
7. |
In Czech a shorter version by R. Bartuch appeared 1878 in the journal Kvas (Ferment) and a full one 1884 by K. Nademlejnský in the journal Pivovarské listy (Brewing Letters). |
|
8. |
He is mainly remembered as the person behind Rudolph's II invitation to Tycho Brahe to come to Prague. For a brief introduction in English to the period, including Hagecius' significance, see Smolka, J. ‘The Scientific Revolution in Bohemia’. In: Porter, R. and Teich, M. (Eds.) (1992) Scientific Revolution in National Context. (Cambridge), 210-39. |
|
9. |
This is dealt with by Drábek, P. ‘Medicínské aspekty v Hájkove knízce o pivu’ (Medical aspects in Hájek's booklet on beer). In Drábek, P. (Ed.) (2000) Tadeáš Hájek z Hájku (Prague), 93-4. Drábek points out that Johann Brettschneider-Placotonus, a physician from Danzig (Gdansk), published 1551 a book on beer De natura cerevisiarum et de mulso, which Hagecius did not know. |
|
10. |
See Preface in Richardson's Statical Estimates. |
|
11. |
Paupie, F.A. (1794) Die Kunst des Bierbrauens physisch-chemisch-oekonomisch beschrieben, Vols. 1-2 (Prague); after the author's death a one-volume second edition divided into three parts was published in 1820. The first and second parts were identical with the text of the first edition. The third part based on Poupe's notes was arranged by J. Sušický. Poupe had in mind to publish his treatise also in Czech which was his mother tongue. But he had to give up the idea because, as he wrote, there was insufficient interest. His second work Versuch einer Grundlehre der Bierbrauerei etc. appeared 1797 in Prague. Finally, his third contribution was written in Czech: Pocátkové základního naucení o varení piva etc. (Fundamentals of Brewing Introductory text etc.) (Olomouc, 1801). |
|
12. |
Cf. ‘Praktischer Unterricht von dem Nutzen des Thermometers und der Bierwage bei der Bierbrauerei nach 16 jähriger Erfahrung’, third part, 143. |
|
13. |
[Ch. P. F.] Erxleben, 1818 Uiber Guete und Staerke des Biers, und der Mittel, diese Eigenschaften richtig zu wuerdigen (Prague). |
|
14. |
Ibid., 69. |
|
15. |
Balling, K. (1843) Die saccharometrische Bierprobe (Prague). Offprint: Encyklopädische Zeitschrift des Gewerbewesens. I further draw on his 1846 Die saccharometrische Bier- und Branntweinmischprobe (Prague) and his classical 1845-7 Die Gährungschemie wissenschaftlich begründet und in ihrer Anwendung auf die Bierbrauerei, Branntweinbrennerei, Hefenerzeugung, Weinbereitung und Essigfabrikation praktisch dargestellt, Vols. 1-4 (Prague). The second edition appeared in 1854-5, and the third in 1865. |